There are three types of nuclear radiation. Alpha particles are energetic fast helium nuclei beta particles are smaller and have half the charge being energetic electrons or positrons only the gamma particles are photons ie they.
What Is The Difference Between Alpha Beta And Gamma Rays
They differ in mass energy and how deeply they penetrate people and objects.

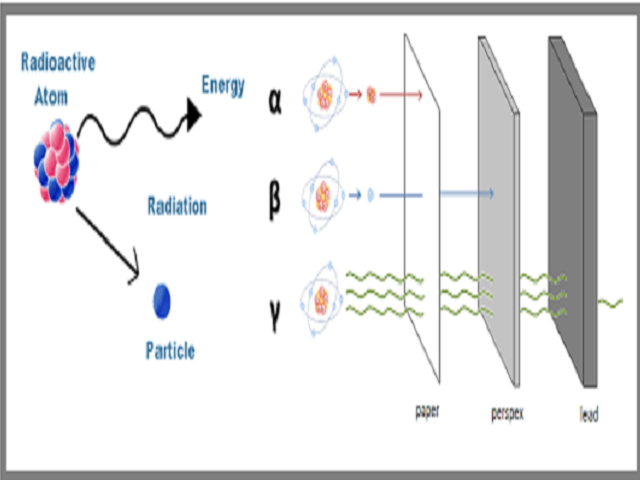
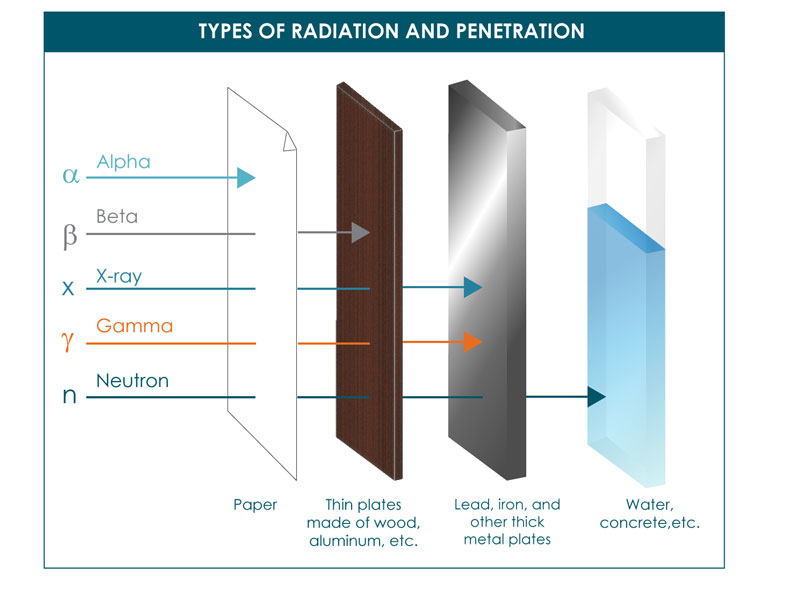
. Nuclear radiation comes from the nucleus of an atom. They have high energy typically in the MeV range but due to their large mass they are stopped by just a few inches of air or a piece of paper. Beta particles can be dangerous and any contact with the body must be avoided though their ionization power is low.
Beta beta - Fast moving negatively charged particle attracted to the positive plate. Beta radiation consists of free electrons or positrons at relativistic speeds which are termed beta particles. An unstable atomic nuclei loss its energy by emitting radiations such as alpha rays beta rays and gamma rays by a process called radioactive decay.
Beta particles have a higher penetration power when compared to alpha particles and can travel through the skin with ease. Alpha rays have poor penetration power. Gamma radiation is a sort of invisible very high-energy light.
The crucial difference between alpha beta and gamma particles lies in their charge constituent. There are three major types of radioactive decay. Gamma rays are often emitted along with alpha or beta particles during radioactive decay.
Gamma rays are similar to visible light but have much higher energy. There are four major types of radiation. They carry a single negative charge.
Alpha alpha - Slow moving positively charged particle attracted to the negative plate. The emission of alpha and beta particles leave the daughter nucleus in the excited state which in turn emits one or more Gamma-ray photons in single or successive transitions. Substances that give out radiation are said to be radioactive.
Since gamma rays penetrate more deeply through the body than alpha or beta particles all tissues and organs can be damaged by sources from outside of the body. Alpha decay involves the loss of a helium nucleus beta decay concerns protons turning into neutrons or vice versa and gamma decay involves the emission of energy without changing the original atom. For the moment well say that alpha and beta radiation consist of tiny particles much smaller than an atom.
This video explains about Alpha radiation. They are more penetrating than alpha particles but. These particles consist of two protons and two neutrons and are the heaviest type of radiation particle.
On the contrary gamma particle has no charge and so is neutral. Alpha decay beta decay and gamma decay. The particles produced by radioactive decay ie alpha particles beta particles and gamma rays are considerably different with distinct physical chemical and.
Beta particles electrons are much smaller than alpha particles. 11 rows Beta radiation is the producer of fast moving electrons and can penetrate further in comparison. There are three primary types of radiation.
Alpha is a positively charged particle beta is negatively or positively charged. Hence it is the electron that is emitted by the nucleus at a rapid pace. Only sufficiently dense shielding andor distance from gamma ray emitting radioactive material can provide protection.
They move incredibly fast perhaps thousands of kilometres per second. Unlike alpha and beta particles which have both energy and mass gamma rays are pure energy. Three types of radioation - Alpha Beta Gamma.
Alpha radiation is the least penetrating. Alpha beta neutrons and electromagnetic waves such as gamma rays. Alpha - these are fast moving helium atoms.
Difference Between Alpha Beta and Gamma Particles Definition Mass Electrical Charge Effect on the Atomic Number Change in the Chemical Element Penetration Power Ionizing Power Speed Electrical and Magnetic Fields Conclusion. Since the gamma rays are emitted by the daughter nucleus emission of gamma rays for the emission of alpha and beta particles. It can be stopped or absorbed by a sheet of paper.
A substance with such an unstable nucleus is called the radioactive substance. Beta - these are fast moving electrons. Difference between Alpha Beta and Gamma rays Radioactivity is the act of emitting radiation spontaneously and is done by an atomic nucleus unstable to attain a more stable configuration by.
Alpha beta and gamma are the first three letters of the Greek alphabet. Alpha particles carry a positive charge beta particles carry a negative charge and gamma rays are neutral Explanation. The first is an alpha particle.
Gamma rays are a radiation hazard for the entire body.
Difference Between Alpha Beta And Gamma Particles Definition Properties Emission Mechanism Applications
What Is The Difference Between Alpha Beta And Gamma Radiation Quora
0 Comments